Share your coffee stories with us by writing to info@comunicaffe.com.
Drs. Joshua Méndez Harper and Christopher H. Hendon discuss the chemistry and physics behind the electrification of coffee during grinding, its effect on extraction, and strategies to mitigate it. All images are adapted from their recent academic publication “Moisture-Controlled Triboelectrification During Coffee Grinding,” published in Matter.[1] Below we share their consideration with an introduction by Chelsea Dubay, Curriculum Director.
About coffee preparation
by Chelsea Dubai
“When I first became a barista, I was captivated by the sheer amount of information available on how to prepare coffee well. Asking “how” to do something— how to brew the most vibrant, consistent espresso, for example—turned up dozens of approaches and considerations, both in person and online.
Asking “why” we use certain techniques turned up at least a handful of working theories, some based on empirical evidence and others based on anecdotal experience. While all of these (sometimes overlapping, sometimes conflicting) recommendations inspired curiosity and experimentation, I often felt overwhelmed and struggled to identify the best techniques to put into practice.
Today, whenever an innovation or breakthrough triggers a reconsideration of the knowledge we reference and skills we use across the specialty coffee industry, I find myself reflecting on similar questions. Now, though, my focus is that of a trainer and curriculum developer: Where do “best practices” come from, and what makes them “best”? Early in my coffee career, best practices seemed to be techniques that achieved some minimal threshold of mainstream adoption—or were endorsed by certain industry celebrities. Today, however, we are fortunate to have access to an increasing number of research studies that guide how we think about coffee preparation.
Take the preparation breakthroughs of the past few years: Who could forget the 2016 study from Uman et al. that demonstrated the benefits of chilling coffee before grinding?[2]
Or the 2020 study from Cameron et al. that explored how to manipulate grind settings and water pressure to reduce the amount of coffee we needed to prepare espresso, all while improving beverage consistency?[3] Sometimes, research inspires new practices (I think about the flash-chilling trend that swept through the competition space after the 2016 study made headlines). In other cases, techniques that felt effective behind the bar are affirmed through the empirical backing that comes from these studies.
This illuminating piece from study authors Joshua Mendez Harper and Christopher Hendon is an example of the latter, explaining their recent research into the role of moisture in mitigating static electricity while grinding coffee.
They found that adding a spritz of water to beans before grinding not only resulted in less grinder retention and cleaner workstations, but also reduced clumping in the coffee bed, decreasing the variability of extraction and resulting in more consistent, reproducible flavors in the cup. The study adds another layer of credibility to the Ross Droplet Technique, a long-discussed practice in enthusiast circles that involves stirring a very small amount of water into a dose of coffee beans before grinding.
How will this research show up in our collective barista practice moving forward? Only time will tell as professionals continue to test the technique and its operational feasibility for themselves—which, as you’ll read, the authors encourage you to do”.
It’s electric: understanding—and reducing—static electricity during grinding
by Drs. Joshua Méndez Harper and Christopher H. Hendon
The act of grinding coffee can generate large amount of electrostatic charge, which not only profoundly affects cup quality, but speaks to some of the oldest unsolved mysteries in material science.
“Consider words used to describe black tea grown in India: you won’t find these words printed on your bag of PG tips, but they are indispensable tools for the brokers and blenders in the Indian tea industry who work to ensure that your daily cup tastes just right. Most black tea on the global market comes predominantly from former European colonies, from East Africa to Southeast Asia. In these places, tea is plucked, pruned, fermented, dried, rolled, sorted, and packaged by hand, on plantations. From plantations, tea is shipped to auction centers in former colonial port cities like Kolkata, Colombo, and Mombasa, where brokers taste and evaluate it before selling it in public auctions.
Kolkata is the heart of the Indian tea industry. Brokers here evaluate over half of all of the tea produced in North India. Millions of kilograms pass through the hands, under the noses, and across the tongues of these brokers. Tea brokerage is a specialized job. Membership in social clubs, facility with English, European dress, and athletic prowess are all seen as signs of one’s qualification.
On a Tuesday morning in 2009, I joined a broker on the top floor of Nilhat House, India’s oldest auction center. Mr. Pal, a tall, avuncular man, was lecturing apprentice brokers about how to properly evaluate tea in order to give it a valuation price for the weekly auctions that his firm oversaw. A group of assistants quietly brewed dozens of tea samples, carefully timing the steeping of leaves with British-made white enamel clocks. Mr. Pal waxed about tea’s remarkable variability. “It’s an agri-product,” he stressed, “so this is a natural variability.”
A broker’s expertise is measured by his—and it is nearly always his—ability to corral tea’s tastes, smells, looks, and textures into a few words, drawn from a fixed glossary. For example, here is how another broker, Mr. Dutta, evaluated an invoice of tea: He slurped one tea, then the next. “Tippy clonals still have fair make. More emphasis on sorting would be of benefit.”
He smelled a pile of steeped leaves, then slurped the liquor. “Mixed. Fannings are acceptable. Clonal has brightness and character, but quality is not there.” He slurped another cup. “A little short in appearance and also not entirely clean. Bloom is lacking.” Mr. Dutta poured the dry leaf of the next invoice onto a piece of cardstock. He shook it back and forth, giving it a few flicks with the back of his fingertips, before bending the cardstock in his hand and funneling the tea back into the bag.
Printed on the top of each sheet of the cardstock that Mr. Dutta used are the words, “It pays to make good teas.” This phrasing is instructive: Brokers do not see themselves as merely separating the “good” from the average or subpar; they explicitly understand their work as one of active making, of bringing good teas into being. For them, qualification “pays”—it is remunerative to them, to sellers, and to buyers.
In many ways, brokers are aesthetic experts, not unlike storytellers or visual artists. They must hone an ability to craft subjective experiences of taste into words and numbers. As much as numerical price, it is words like make, character, and brightness that indicate a tea’s quality. A list of some 150 terms is published by the Calcutta Tea Traders Association and rendered on glossy posters that adorn brokerage firm walls. These “teawords” are all tools for discerning the quality of tea.
In an interview with me, a Kolkata broker I’ll call Mr. Chetal described learning to be a broker in this way: “You train and train, and one day you realize that you are training yourself through your own processes of experimentation.” He was referring to a broker’s need to creatively combine his sensory faculties, his familiarity with the terms in the glossary of teawords, and his knowledge about the dynamics of the market. For tea brokers, experimentation with words is at the same time experimentation with things and bodies.
As Mr. Chetal explained:
If I said stewy to you, what would you think? It has the characteristics of stew, right? Thick, cloudy. But no, that’s not what it means at all! The meaning is much more exact. It refers to the exhaust temperature. It means that [a tea] was fermented at too high a temperature, that it over-fermented; it therefore has become soft.
In his work on the terms used to describe wine, anthropologist Michael Silverstein noted that such words act as a kind of connective tissue, linking nodes in a product’s trajectory from production to consumption. Teawords such as stewy and soft connect taste, smell, and appearance in the tasting room to events in the field and factory—and they work in combinations: Mr. Chetal’s hypothetical stewy tea was also soft. A soft tea lacked briskness (a liquor that is alive, like fresh spring water). It also lacked brightness (a liquor and leaf whose colorful pop would be visible even when mixed with milk). Seemingly straightforward words like cheesy, minty, and fruity do not reference the sensations of cheese, mint, or fruit. These words signal different “taints” in the teas imparted during storage or transport. Biscuity is a pleasant characteristic, and shotty teas are not “shoddy” at all, but well made. Spongy leaves are actually flat and flaky. An earthy taste, while desired in some wines, indicates that a tea was stored in damp conditions, while winey liquors are over-fermented, albeit in sterile conditions. Mr. Chetal continued,
“The language of tea is an intra-trade language. Tea is unlike wine, whose language is applied toward the consumer. [Wine] terms are evocative, finely tuned, and pleasing. They generate emotion.” Tea’s terminology is only an expert language. Consumers who drink tea will likely never be aware that teawords exist. The origins of teawords lie as much in colonial agronomic science as in a colonial economy of prestige, and it is the historical meeting of these two where I have focused my anthropological research”.
Negotiating the language of quality
In 1911, the Indian Tea Association, or ITA, established the Tocklai Experimental Station in Jorhat, Assam. Early Tocklai experiments, in areas such as pest management, were oriented to maximizing the quantity of tea that plantations could produce. But by 1932, a global economic depression was under way, and consumer buying power was at an ebb. Facing shrinking demand, tea plantations across the British Empire agreed to curb production and focus on making what they termed “quality tea.”
It was in the context of this industry-wide turn to quality that a chemist and meteorologist at Tocklai, C. R. Harler, first proposed a standard glossary. Harler had observed brokerage practices, and he became frustrated that no one in the industry used a common language to describe the characteristics of tea. He elaborated his critique in a 1932 article, in which he complained not only that brokers’ language was unstandardized but also that: there are a good number of terms used by individual tasters which convey little or nothing to the average planter.
Thus, in one case, a tea infusion was described as tasting like a “bandsman’s tunic.” Such an expression connotes unpleasantness, and may denote sweatiness, but gives no definite guidance to a planter who wants to trace a shortcoming in his tea to some incorrect factory procedure.
Phrases like “bandsman’s tunic,” while long on poetics and certainly descriptive, were metaphorical. Their material referents lay outside the chain of sites along which tea traveled.
Harler argued that it was possible to link teawords to production processes. He proposed not just to narrow the number of descriptive terms to be used, but to identify which chemical constituents of tea were responsible, either wholly or in part, for qualities like sweatiness— or rawness, briskness, pungency, strength, color, or thickness. In 1934, the ITA submitted Harler’s glossary to the Tea Brokers’ Association of London for comment; the tea brokers agreed to reduce their evaluative language to a finite number of terms.
They came up with a revised glossary, which was forwarded to the ITA’s Calcutta office for further refinement, in consultation with Tocklai scientists. After that round of revision, the glossary was returned to the Tea Brokers’ Association for final revisions, followed by approval by the ITA’s London- based scientific advisory committee. Word by word, representatives from the plantation, brokerage, and scientific sectors settled on terms for describing the qualities of dry leaves, steeped leaves, and tea liquor.
For example, the term bakey refers to the taste of infused tea liquor. In his original glossary, Harler defined bakey as “a slightly high fired tea.” The 1934 revision by the ITA London Committee and the Tea Brokers’ Association revised that definition to: “Defective or faulty firing and sometimes slightly over fired.” Once the glossary was returned to scientists at Tocklai, they amplified the definition further, to add the phrase: “Certain instances of ‘bakeyness’ have been associated with bacterial infection.”
When Tocklai’s revisions were reviewed back in London by the Tea Brokers’ Association, they deleted the final sentence about “bacterial infection.” In the final glossary, published in 1938, bakey is defined as follows: “Faulty firing, not necessarily at too high a temperature but often due to leaves being too long or too thickly spread in the dryer.”
Harler’s efforts brought forth a debate about the definition and boundaries of “quality.” Take bakey: Tocklai scientists wanted to link a “bakey” sensation to bacteria, but brokers and planters consistently rejected such direct causal references. According to brokers and planters, in order to be effective tools for maximizing quality, teawords needed to be standardized enough to be mutually intelligible, but not so standardized that they became distinct categories of “goods” and “bads.” Quality was not so stable. It had to be constantly reproduced and rediscovered in practice, including linguistic practice.
As the revisions were going on, in 1935, the ITA appointed Cambridge University biologist Frank Engledow to form a commission of enquiry to identify a way forward for the industry in the context of depression-induced crop restrictions, looming world war, and a rising tide of anticolonial sentiment. India would become independent by 1947, but even then, there was no clear sign that British capital would be expelled from the country. Engledow’s mission, then, was to find a way to ensure the sustained dominance of British capital in the subcontinent after the formal end of empire: he was charged with ensuring the continued market dominance of tea over other stimulants like coffee and cocoa.
The Engledow Commission’s survey of plantation owners and managers revealed that the “improvement of quality” remained the membership’s highest priority, but Engledow, the biologist, remained skeptical. After all, what counted as quality was still determined in large measure by a group of brokers whose practices remained largely illegible to science. Imperfect as it was, the new glossary had the potential to link British scientific practice (represented by Tocklai), British aesthetic practice (represented by tea brokers), and British productive practice (embodied by the plantation complex).
To do this, the Engledow Commission proposed “a new specification of quality,” in which the professional broker would become “a key-member of the scientific staff.” For the commission, the objective of scientific investigation should be “to connect cause and effect”: to understand the relationships between the sensory and material qualities of tea, as assessed by brokers, and notions of greater or lesser quality, as reflected in market price.
Engledow believed that this could be done if brokers used not just a controlled lexicon, but only five descriptive words: color, strength, pungency, quality, and flavor. With this limited lexicon, the brokers’ body would become a kind of laboratory instrument. Its measurements would be calibrated to a known standard set by practitioners of the new science of industrial chemistry; these five words each correlated to a sensory experience of taste, look, and feel. Those experiences indirectly pointed to different aspects of plantation field and factory production.
Between the 1940s and 1950s, chemists worked with a panel of professional brokers to use these five terms, along with a strict set of criteria set out by Engledow. For example, a tea’s “colour” could be judged to be equal to, better, or worse than a known standard, using a formal grading system. Engledow’s goal was to make the monetary pricing of tea entirely subject to scientific scrutiny—to slowly eliminate any hint of aesthetic sensibility from the market. In fact, he ultimately wanted to eliminate brokers altogether. In the end, this mission failed: Engledow’s five words never replaced the larger glossary, and science has not supplanted brokerage. But this work did help make the brokerage practice look more like a laboratory practice.
The brewing of tea in tasting rooms in Calcutta and London came to be more standardized across different brokers and brokerage firms. It began to be synchronized by precise clocks. Weights of tea and volumes of water, even the crockery out of which tasters slurped their tea, were all now more tightly articulated, all in the aim of assessing—and stabilizing—quality.
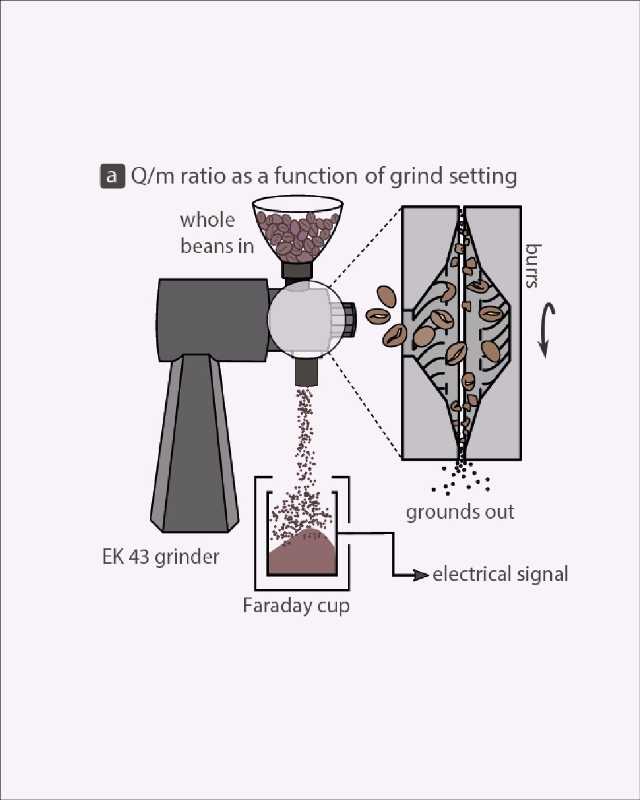
Understanding static: where and how?
If we wish to address electrification during grinding and its effects on brewing, we need to understand where and how the electricity is generated. The act of grinding, i.e., fragmentation and friction, certainly catalyzes charging, but the generation of static electricity is ultimately controlled by a material’s microscopic surface chemistry.[10] The complexity of material surface properties, and how environmental conditions influence these properties, is in no small way responsible for our poor understanding of static electricity in general.
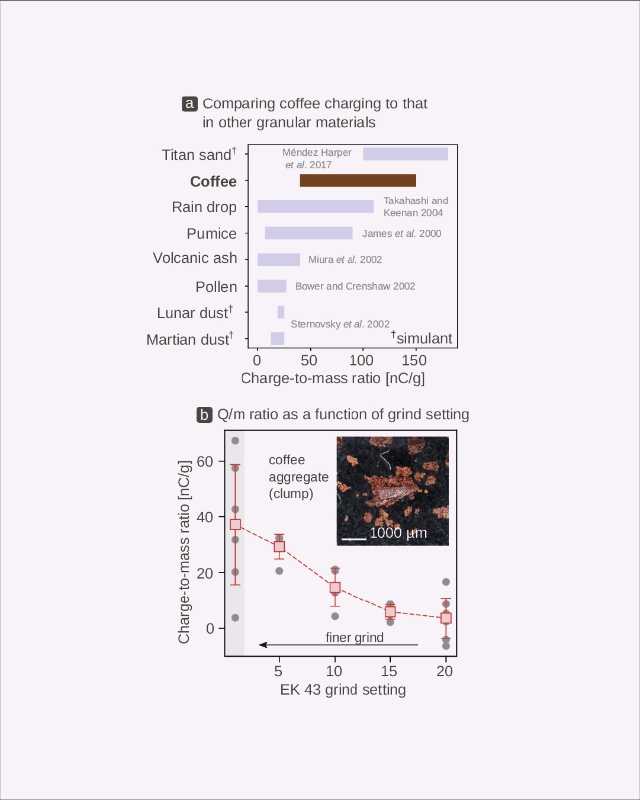
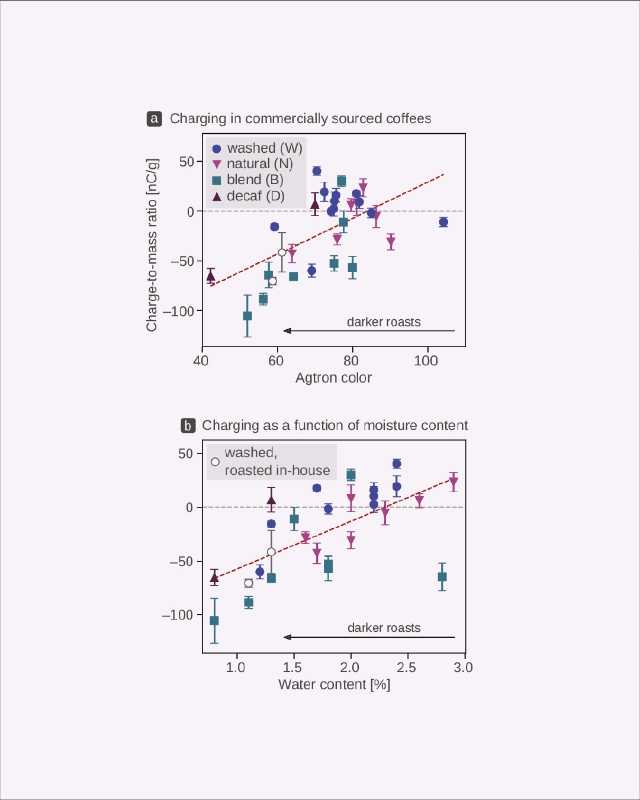
For example, a surface electrified at a high temperature may gain a wildly different electric charge to the charge obtained by a seemingly identical material exposed to cold. To understand this complexity specifically in coffee, we explored the charging of around 30 different commercially sourced coffees when ground (Figure 3) and observed a large scatter in the data regardless of whether we were looking for a relationship between the static generated and the roast color (Figure 3a) or the static generated and the water content of the coffee (Figure 3b).
Although there was some correlation between these factors and the electric charge generated (light roasts charge positively, whereas darker ones gain negative charge), these relationships are statistically “weak.” This may be because of the compound effects of things like variability across growing, processing, and roasting conditions—all the characteristics of a green coffee and its roasting ultimately change its final chemical composition, which then influences the generation of static electricity during grinding.
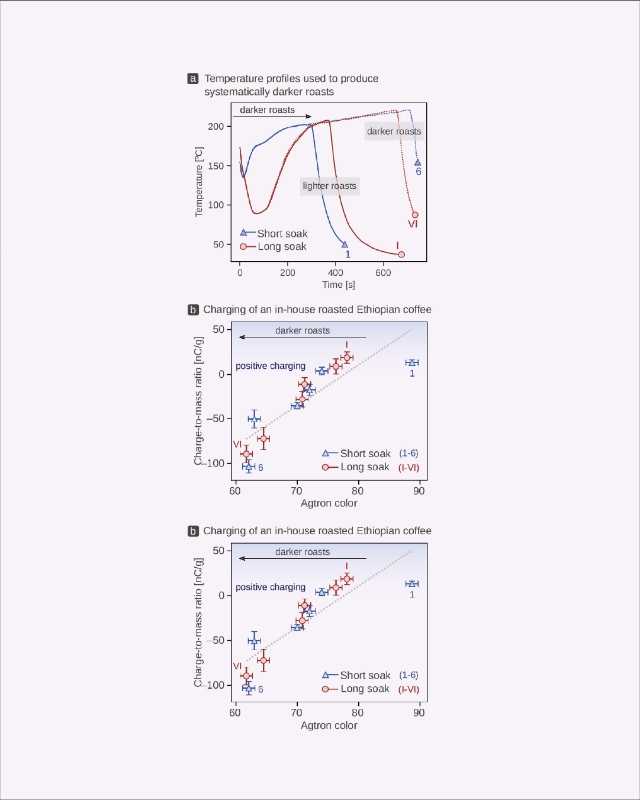
To better test these relationships, we took a single green coffee—a washed Ethiopian Yirgacheffe without any defects—and developed two sets of roast profiles so that we could isolate the effects of color and residual water content on static electricity (Figure 4). The sets differed in the length of the “soak-in” period.[11] We then roasted the coffee with both of these profiles by systematically increasing the total length of the roast as well as the maximum temperature (Figure 4a).
When comparing the shortest (i.e., lightest) of these profiles to the longest (i.e., darkest) of these profiles, we observed behaviors similar to those seen in commercially sourced coffees: the darker (drier) roasts charged negatively, whereas the lighter (with more residual moisture) roasts gained positive charge.
Again, we found that electrification correlates weakly to color (i.e., there is a lot of “scatter” in the data), with a transition from positive to negative charging at Agtron colors of 70–80 (Figure 4b). In terms of moisture, however, we saw a stark departure from the behavior observed in commercially sourced coffees: although a transition from negative to positive charging still occurred once a coffee had a water content of approximately 2%, we found the relationship between moisture and charging to be exponential, not linear (Figure 4c). This kind of relationship has been observed in other processes, including a study on bananas,[12] and we found a much stronger correlation.
This means that the residual internal moisture of a roasted coffee is an excellent predictor of the resulting electric charge, regardless of color. In other words: roast color, while providing a touchpoint for flavor, does not yield sufficient information about the chemical composition and the static electricity it generates, but internal water content does appear to be a primary factor in terms of what static is generated (and how much), making it a useful predictor.
Managing static: just add water
Having defined the parameters that lead to charging in coffee, we now turn to strategies to deal with static electrification. One may be tempted to use more grounded metal components in the construction of grinders with the hope that some of the charge becomes funneled away (much like a grounded lightning rod serves to earth discharges during thunderstorms). A problem here is that dry coffee is an insulator, meaning it does not readily conduct electrical charge.
So, while the charge on the side of a particle in direct contact with grounded metal will be neutralized, the charge on particle surfaces at any distance from these grounded components will remain in place (Figure 5a). Because of this, grounding alone may lead to more problems than solutions: even at a distance, trapped charge will be attracted to grounded surfaces, causing particles to stick to them. While grounding strips may keep particles from fluttering away, they can potentially increase retention (Figure 5b).
There are two possible solutions to the problem of trapped charge on insulating particles: either increase the mobility of the charge (by making the particles more conductive, say) or “inject” charge of the opposite polarity into the mass of ground coffee to neutralize immobile charge. The first approach to electrostatic charge reduction (increasing charge mobility) has been implemented by the coffee community (baristas in particular) for a long time. Referred to occasionally as the “Ross Droplet Technique” (RDT)[13] by baristas and enthusiasts alike, a small amount of water is added, using a spray bottle or dropper, to whole beans before grinding.
This addition of free water (i.e., water not contained within the coffee) theoretically increases the conductivity of particle surfaces, activating pathways for trapped positive and negative charges to recombine or to flow toward grounded surfaces. Beyond increased recombination, added water may change the surface composition of coffee through electrochemical reactions, perhaps leading to more inefficient charging in the first place. Our next step in understanding the static electricity generated through grinding was to explore whether or not the addition of water reduced static.

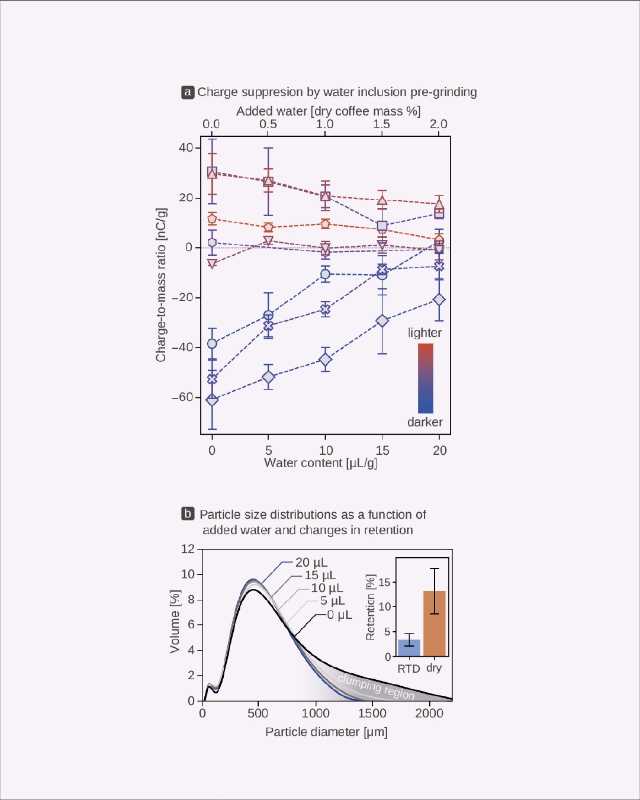
To do this, we ground a subset of the same commercially sourced coffees[14] along with an increased amount of water, from 0 to 20 μL per gram of whole beans to measure changes in both the presence of static and clumping (“particle charge” and “particle distribution,” respectively, Figure 6). Even at very low water contents, we noticed a marked decrease in electrification (Figure 6a)—but at the highest content of 20 μL/g, the addition of water reduced the charge-to-mass ratio by half. Also, as charge decreased, so did the capacity for coffee particles to clump: this “deaggregation” resulted in a shift in particle size distributions toward smaller diameters, reflecting clumps breaking up into individual grains (Figure 6b). Did less static actually mean less retention in the grinder? Yes: the addition of 10 μL of water per gram decreased retention for a dark roast from over 10% to around 2.5% (inset Figure 6b).
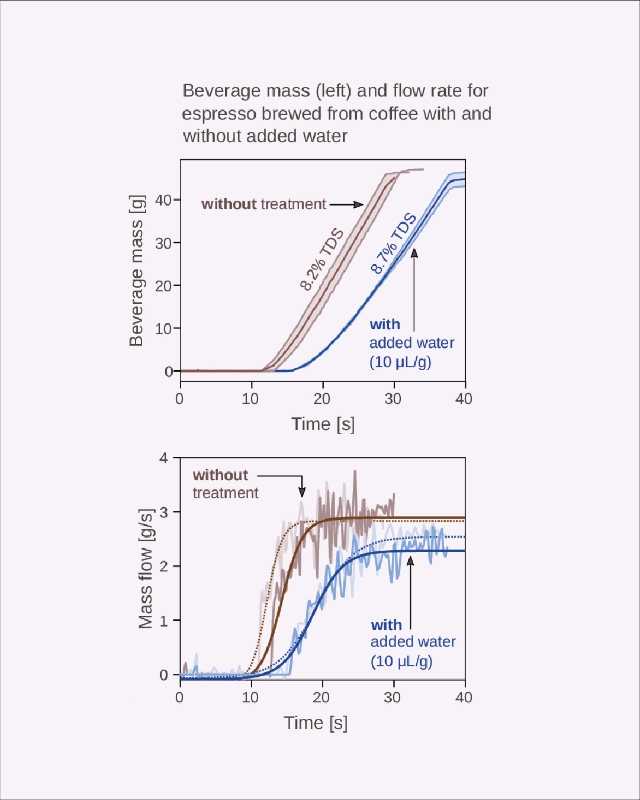
While these effects felt a little obvious, our step was to explore what impact the addition of water during grinding had on brewing. To answer this, we prepared espressos to assess their time-dependent extraction characteristics in relation to the amount of water added to the coffee before grinding.[15]
When we added 10 μL/g of water before grinding, we noticed two important things: espresso shots took nearly 50% longer to reach the desired volume out than when brewed with coffee that hadn’t received a water spray before grinding. They also demonstrated a markedly decreased flow rate (Figure 7). We noticed a change in cup concentration, too: the espresso prepared with dry ground coffee yielded 8.2% total dissolved solids (% TDS), but the espresso prepared with the “wet” coffee produced a cup with 8.7% TDS.
From a physics perspective, we believe this change in TDS is due to an increased bed density in the portafilter, as the fines and boulders would not be electrostatically attracted to one another. This means that, as clumps fall apart, fines are able to efficiently “pack” the bed, allowing water to more uniformly contact coffee during the shot. In their 2020 paper, Cameron and others[16] suggest that a finer grind may yield uneven and variable extraction (due to uncontrolled bed porosity), but our findings suggest that the addition of water to roasted coffee before grinding may provide a direct remedy to this problem by homogenizing the bed.
The increase in concentration afforded by added water (~10–15%) should certainly affect taste, but also has important cost-saving implications for the coffee industry, which is worth US$343.2 billion or 1.5% of the US gross domestic product. And these experiments have only highlighted the changes in TDS and flow rates for a single grind size! There’s more to learn about how RDT impacts coffee preparation using other brew ratios and grind settings. If you are comfortable with adding small amounts of water to your grinder, we hope this inspires you to explore these variables yourself.
The fact that so many coffee professionals are familiar with the idea of adding a squirt of water to coffee before grinding, together with the data presented here, suggests that the practice resolves problems involving clumping, channeling, and poor extractions. However, as too much water may promote caking or corrosion within the grinder, there has been recent interest in developing “dry” electrostatic dissipation techniques. These methods generally involve the production of free ions—that is, negatively or positively charged gas molecules that neutralize charges on coffee grains. We investigated this approach in another study, and found that its effectiveness depends greatly on the coffee being ground (namely, roast color and moisture content) and at what point the ions are added (e.g., at the output of the grinder or within the grinding cavity itself).[17]
This variance stands in contrast to the Ross Droplet Technique, which reduces the static charge regardless of coffee color or residual water content (see Figure 6). Beyond the application of water and ionization, there may be other charge-reduction strategies, like selecting burr materials with anti-static coatings or designing grinders with charge-dissipative geometries (like the static wicks on the wings of aircraft); exploring these approaches is something worth doing in detail.
So far, electrostatic charging during coffee grinding has been cast in a rather negative light, but there may be some benefit to be gained from this pervasive electrification. Perhaps this charging behavior reveals chemical and physical qualities unseen by other metrics; after all, as we have said above, contact and frictional electrification arise from these very material properties.
In fact, such ideas have been pursued vigorously in other fields: volcanologists, for example, have spent the last 20 years linking particle charging to the amount of ash, steam, and other compounds erupting from active volcanoes.[18] Other researchers have shown that the static caused by certain plastics is sensitive to contaminants in liquids and gasses, allowing for the detection of harmful chemicals in the environment.[19]
Could similar methods be used to pinpoint defects in whole beans or monitor the composition and homogeneity of blends? The possibilities of static as a diagnostic tool are exciting but virtually unexplored in the context of coffee.
While the application of electrochemistry to coffee may seem like a fairly niche—albeit exciting!—area of study, the insights we have gained from our experiments extend well beyond the cup, impacting our knowledge of electrification processes associated with pharmaceutical powders, volcanic eruptions, and even the sand dunes of Saturn’s moon Titan.[20] Understanding charging during coffee grinding not only aids in the pursuit of the tastiest espresso, it also brings us closer to resolving long-standing questions in material science, engineering, and geophysics”.
References
[1] Joshua Mendez Harper, Connor S. McDonald, Elias J. Rheingold, Lena C. Wehn, Robin E. Bumbaugh, Elana J. Cope, Leif E. Lindberg, Justin Pham, Yong-Hyun Kim, Joseph Dufek, and Christopher H. Hendon, “Moisture Controlled Fracto- and Triboelectrification During Coffee Grinding,” Matter (2023): https://doi.org/10.1016/j.
[2] Uman et al., “The Effect of Bean Origin and Temperature on Grinding Roasted Coffee,” Scientific Reports 6 (2016), https://www.nature.com/
[3] Cameron et al., “Systematically Improving Espresso: Insights from Mathematical Modeling and Experiment,” Matter 2, no. 3 (2020), https://doi.org/10.1016/j.
[4] Paul Iversen and Daniel J. Lacks, “A Life of Its Own: The Tenuous Connection between Thales of Miletus and the Study of Electrostatic Charging,” J. Electrost. 70, no. 3 (2012): 309–311, https://doi.org/10.1016/j.
[5] Daniel J. Lacks and R. Mohan Sankaran, “Contact Electrification of Insulating Materials,” J. Phys. Appl. Phys. 44, no. 45 (2011): 453001, https://doi.org/10.1088/0022 3727/44/45/453001.
[6] Corrado Cimarelli, Sonja Behnke, Kimberly Genareau, Joshua Mendez Harper, and Alexa R. Van Eaton, “Volcanic Electrification: Recent Advances and Future Perspectives,” Bull. Volcanol. 84, no. 8 (2022): 78, https://doi.org/10.1007/
[7] Tobias Steinpilz, Kolja Joeris, Felix Jungmann, Dietrich Wolf, Lothar Brendel, Jens Teiser, Troy Shinbrot, and Gerhard Wurm, “Electrical Charging Overcomes the Bouncing Barrier in Planet Formation,” Nat. Phys. 16, no. 2 (2020): 225–229, https://doi.org/10.1038/
[8] To maintain consistency with other works, we report electrification as charge-to-mass (Q/m) ratios with units of nano-Coulombs per gram. A nano-Coulomb is equal to the charge on approximately 6.24 billion electrons.
[9] Firstly, per gram, smaller particles have more extensive surface areas than larger particles. As the surface area involved in frictional interactions increases, so does the potential for triboelectrification. Further, as coffee breaks into smaller and smaller bits, fragments gain additional charge through fracto-electrification—
[10] Daniel J. Lacks et al.: 453001.
[11] There are many ways to manage the internal temperature of green coffee that take both the latent heat of a roasting drum as well as the heat of the burners into account. “Soaking” is a technique where a roaster cuts the ignition of the burners once green coffee has been added to the drum (“charged”) for a period of time before reigniting the burners, changing the rate at which the green coffee’s internal temperature rises.
[12] Rak Dandamrongrak, Gordon Young, and Richard Mason, “Evaluation of Various Pre-Treatments for the Dehydration of Banana and Selection of Suitable Drying Models,” J. Food Eng. 55, no. 2 (2002): 139–146,